
Back |
Interactive 3D PDF of Carnegie Stage 11 |
The interactive PDF below has been very kindly provided by Dr. Jill PJM Hikspoors, Prof. Wouter H Lamers,
Department of Anatomy & Embryology, Maastricht University, Maastricht, The Netherlands
Contact: jill.hikspoors@maastrichtuniversity.nl
Publication: 10.1038/s42003-022-03153-x
*Important* Although some web browsers will allow PDFs to be viewed online, the file must be downloaded and saved to a computer to enable the interactive options.
 |
 |
 |
 |
 |
||||
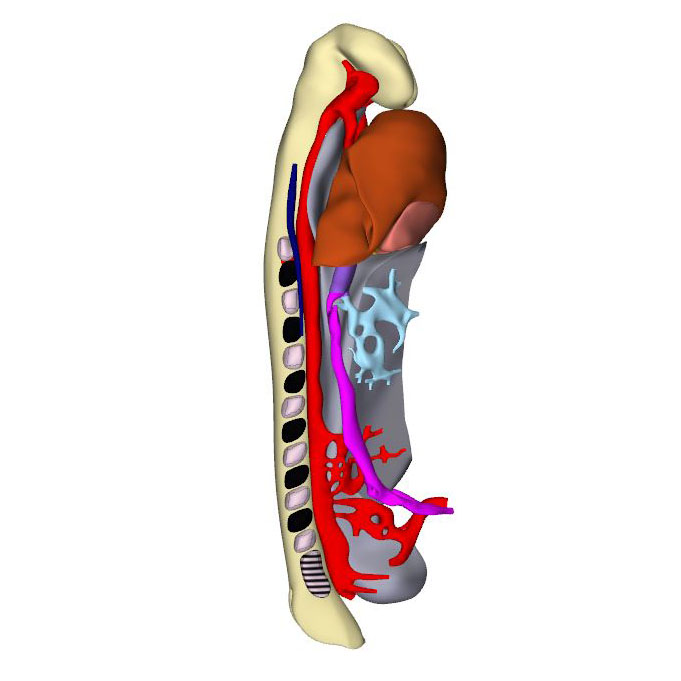
Right-sided view |
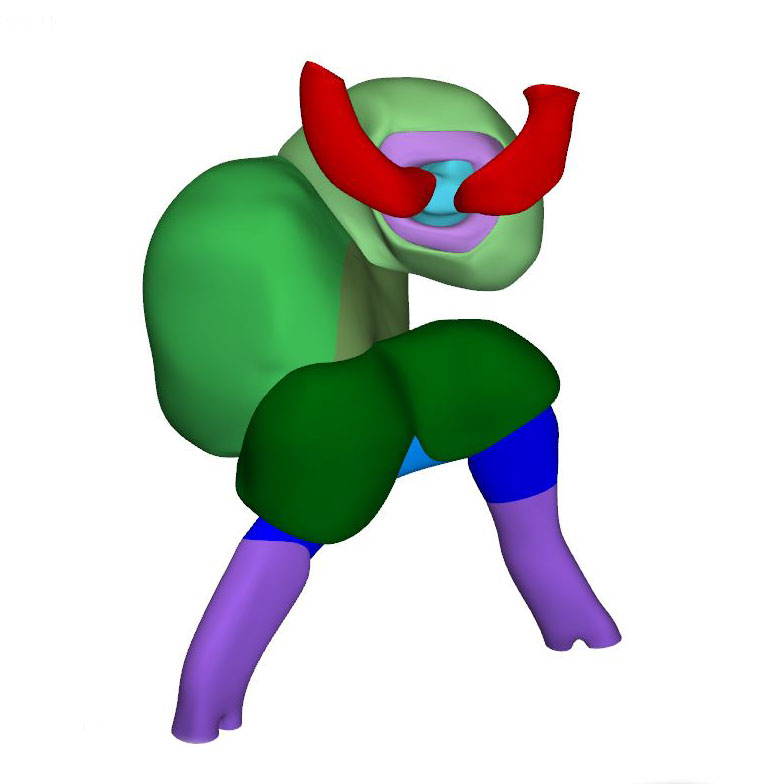
Right dorsal view: Neural tube with its neuropores |
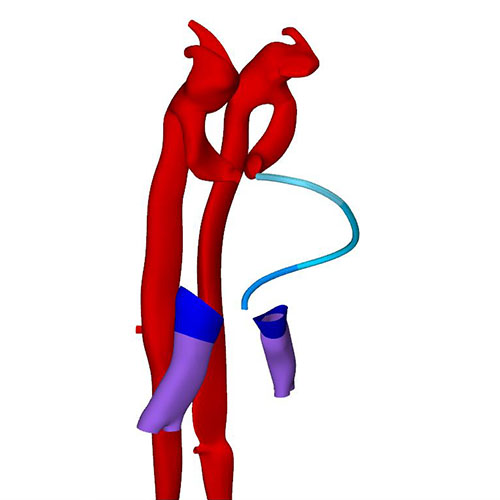
Right ventral view: Cardiac looping |
Right ventral view: Loop wire shows looping pattern |
Dorsal view: Left ventricle is starting to balloon at the outer curvature |
Human embryos reach the 11th Carnegie stage when ~29 days have passed after conception. It has developed 13 pairs of somites. The neural plate has partially transformed into a tube, with its neuropores reaching the mesencephalon cranially, and the somitomeres caudally to somite #13, which is equivalent to vertebral level T2. The pharynx by now extends further cranially and has widened, but has not, as yet, given rise to individual pouches. The pericardial cavity extends between the stalk of the yolk sac and transverse septum caudally, the pharynx dorsally, and the forebrain cranially. The entire cardiac tube, except for its caudal non-myocardial inflow tract, is invested in cardiac jelly and has myocardial walls. The ventricular lumen shows pronounced folds along its longitudinal axis, which represent outward extensions of the endocardial tube that presage the appearance of the ventricular trabeculations in the next stage. At the venous pole, the hepatocardiac channels, formed from the vitelline and umbilical veins, drain into the inflow tract. Together, the hepatocardiac channels and the inflow tract determine the contour of the cranial intestinal portal. The vitelline veins develop on the craniolateral surface of the yolk sac. Although the cardinal veins have begun to form within the embryo, their connections with the venous pole of the heart have yet to form. Coelomic cells have formed two, still separate proepicardial organs at the junction of the hepatocardiac channel and the inflow tract.
The expansion and the medial fusion of the myocardial walls of the atria are indicative of continuing differentiation. The beginning of ballooning of the right atrium, and the leftward transfer of the atrioventricular canal permit the recognition of laterality. This laterality involves differences in both lineage and phenotypic properties of the right and left atria. The apical part of the embryonic left ventricle is also beginning to balloon at the outer curvature of the loop. The outflow tract still bifurcates just ventral to the pharynx into the ventral aortas, which continue dorsally on either side of the pharynx to join both dorsal aortas. The dorsal aortas extend caudally to the dorsal wall of the cloaca and have not yet started to fuse. Near the caudal part of the junction of the midgut and stalk of the yolk sac, an arterial plexus with multiple roots in the ventral wall of the dorsal aorta begins to extend ventrally towards the yolk sac. This plexus represents the earliest stage in the development of the celiacomesenteric trunk. On the left side, one arterial vessel has a markedly bigger diameter than all other components of the arterial network. Slightly more caudal, yet another plexus with multiple roots in the dorsal aortas extends along the cranial wall of the cloaca towards the connecting stalk. This plexus represents the earliest stage in the development of the umbilical arteries.
The appearance of the transverse pericardial sinus in CS10 embryos marks the beginning of cardiac looping. The accompanying rightward tilt of the arterial pole, and the leftward tilt of the embryonic left ventricle, are further overt and early signs of asymmetry in these young embryonic hearts. In mice at a similar stage of development, growth in the left side of the arterial pole, the ventral side of the loop, and the right side of the venous pole, exceeds that in the corresponding opposite sides. These findings suggest that the breaking of symmetry during looping results from the asymmetric distribution of cell-proliferation centers. Due to a higher rate of proliferation and myocardial differentiation of mesenchymal cells in the dorsal mesocardium, and their subsequent insertion into the venous and arterial poles of the heart, the length of the limbs of the cardiac loop increases between the left atrium and the arterial pole, in particular in its cranial outlet segment. At the venous and arterial poles, the heart retains its midline connections with the pharyngeal mesenchyme through the remaining parts of the dorsal mesocardium.
Click an image to download a 3D-PDF. The file must be saved to a computer to enable the interactive options. The 3D-PDFs can be opened on any computer as long as Adobe PDF or equivalent reader is installed.
A 3D-PDF becomes activated by “clicking” with the mouse on the reconstruction.
A toolbar appears at the top of the screen that includes the option “model tree”.
The model tree displays a material list of structures in the upper box, and preset viewing options (cameras) in the lower box. The sequence of items corresponds to that in Supplemental Table 3 of the publication.
The list of visible structures can be modified by marking or unmarking a structure.
To manipulate the reconstruction, press the left mouse button to rotate it, the scroll button to zoom in or out, and the left and right mouse buttons simultaneously to move the embryo across the screen.
A structure can be rendered transparent by selecting that option from the drop-down menu after selecting the structure with the right mouse button.
To inspect a combination of structures, one is advised to build up the composition, beginning with a familiar component, such as a lumen, rather than deleting non-relevant structures one-by-one from a completely reconstructed specimen.
The slicer button in the toolbar allows making cross sections. The plane of section can be adjusted with the offset and tilt options.
The “loop wires” in Supplemental Figures 3-6 of the publication, which are drawn through the center of the endocardial heart tube, emphasize the changing shape of the heart loop during CS10-13.
The side length of the scale cubes is 200 μm.
The preset views correspond to the images shown in Figures 1-10 of the publication.
Note that items that are visible in these views can be altered by marking or unmarking a structure in the model tree.