
Back |
Interactive 3D PDF of Carnegie Stage 14 |
The interactive PDF below has been very kindly provided by Dr. Jill PJM Hikspoors, Prof. Wouter H Lamers,
Department of Anatomy & Embryology, Maastricht University, Maastricht, The Netherlands
Contact: jill.hikspoors@maastrichtuniversity.nl
Publication: 10.1038/s42003-022-03153-x
*Important* Although some web browsers will allow PDFs to be viewed online, the file must be downloaded and saved to a computer to enable the interactive options.
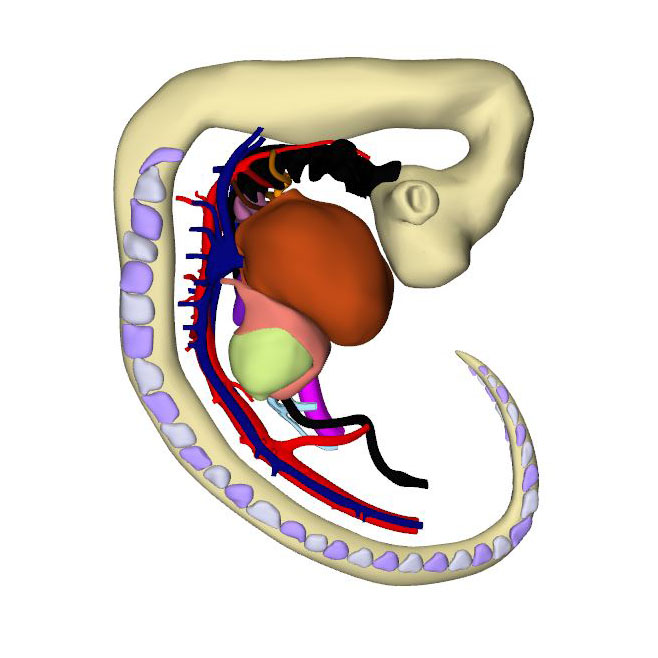 |
||||||
Right-sided view |
Dorsal right view: Venous sinus has myocardium. Sinoatrial node seen as a myocardial cuff |
Dorsal right view: Sinoatrial junction (right) and pulmonary vein (left)
|
Right sagittal view: Interatrial foramen and interventricular foramen |
Right sagittal view:Transparent superior endocardial cushion showing division in the lumen of atrioventricular canal |
Right view: Pharyngeal arch arteries |
Cranial view: Myocardial outflow tract with a fish-mouth appearance |
Left cranial view: Neural crest cells divide aortic sac into aortic and pulmonary portions |
Left cranial view: Neural crest cells transform cardiac jelly into septal and parietal ridges |
Left cranial view: Aortic and pulmonary columns with swellings (in alternating position to neural crest cells) |
Ventral view: Changing position of right ventricle |
The embryo has now been developing for ~34 days subsequent to fertilization. Since the cranial somites are no longer identifiable, we revert to spinal ganglia as our reference for segmental level. The right hepatocardiac channel has become part of the inferior caval vein. The left hepatocardiac channel is still seen in early specimens of CS14 but has regressed in this advanced CS14 specimen. The confluence of the cranial and caudal cardinal veins has substantially increased in diameter on both sides, while both sinus horns have completely myocardialized. The primordium of the sinus node, with an obvious tail, is recognizable as a myocardial cuff at the junction between the right atrium and right common cardinal vein, which itself is now recognizable as the superior caval vein. In mice, the left-sided marker Pitx2c suppresses development of a sinus node along the left common cardinal vein.
The sinuatrial connection, now narrow, is guarded by the venous valves. These valves merge into the spurious septum craniodorsally, and attach in the primary myocardium of the atrial floor caudoventrally. The primary atrial foramen remains surrounded by the atrial extensions of the superior and inferior atrioventricular cushions, with the extension of the superior cushion being a mesenchymal cap on the leading edge of the newly-developing primary atrial septum. The pulmonary vein, which passes between the atrial extensions of the atrioventricular cushions and through the dorsal mesocardium, has now canalized so as to connect with the venous plexuses developing ventral to the lung buds. The rightward margin of the dorsal mesocardium is known as the vestibular spine or, more recently, the dorsal mesenchymal protrusion. The atrioventricular canal itself is surrounded by bilayered primary myocardium that extends in the atrial floor to the root of the pulmonary vein and the base of the right venous valve. This extension is also known as the body of the atrium, but shares its lineage and early growth pattern with that of the atrioventricular canal. The cushions within the canal now divide its lumen into narrow left and right atrioventricular passages, but have yet to fuse.
The trabeculated free walls of the ventricles continue their ballooning. The left ventricle, thus far made up of cardiomyocytes originating in the first heart field only, also expands by recruiting cells from the atrioventricular canal. With the ballooning of the ventricular compartments, it is now possible to recognize the muscular ventricular septum. Cell multiplication at its base and a circumferential growth pattern bring about a sharp boundary between the left and right sides of the septum. Its crest forms the caudal margin of the interventricular foramen, with the inner curvature forming the cranial margin. The myocardium surrounding the interventricular foramen, which is the first component of the second heart field to differentiate, can be stained with monoclonal antibodies that recognize “GlN”, a terminal 3-sulfated glucuronic-acid epitope on macromolecules. The very dense appearance of the myocardium of this interventricular ring also makes possible identification of its components in routine histological sections.
The development of a physical separation between the systemic and pulmonary circulations is known as “septation”. In early embryonic hearts of mice and chicken the blood flow is laminar, which limits its mixing. With the appearance of ventricular trabeculations during CS13-14, conduction velocity through the myocardial walls increases, and the activation of the ventricle changes from a base-to-apex to an apex-to-base sequence. Cardiac pumping, furthermore, switches from a suction, or impedance, to a pulsatile, or piston, mechanism. Because cardiac output increases, vortical patterns of streaming and mixing develop, especially downstream of the relatively narrow and still slowly contracting atrioventricular canal and outflow tract. The temporal correspondence of the increasing functional effectivity of embryonic hearts and anatomical septation, therefore, is not coincidental.
Septation proceeds centripetally from the venous and arterial poles towards the interventricular foramen. Septation of the inflow tracts becomes feasible once the systemic venous sinus and its tributaries are committed to the developing right atrium, and the pulmonary vein is committed to the developing left atrium. This is seen in CS13 embryos. The primary atrial septum begins to form at CS14, followed by functional septation of the atrioventricular canal by the endocardial cushions into left- and right-sided channels. The borders of the interventricular foramen become remodeled eventually into peri-tricuspid and peri-subaortic portions. These are then separated anatomically by the formation of the membranous septum, which closes the middle portion of the initial foramen at CS20. It is the residual primary myocardium in the inner curvature of the heart that becomes modified during these processes. The expression of the GlN epitope in the myocardium surrounding the interventricular foramen facilitates the description of the changes in its shape during the process of septation. Until the end of CS15, however, it remains a flat and round entity, with its borders well described as the primary ring.
Septation of the outflow tract proceeds from the aortic sac towards the interventricular foramen. At CS14, the arteries of the 2nd pharyngeal arch have disappeared, while the arteries of the 6th pharyngeal arch have formed. Although only 5 pharyngeal arches form in amniotes, it remains conventional to describe the ultimate arches as being the 6th entities. The pulmonary arteries have yet to appear in this embryo. Pharyngeal arch arteries 3, 4, and 6 form by vasculogenic differentiation of mesodermal cells from the second heart field, whereas their smooth muscle coat derives from the cardiac neural crest. In mouse embryos this part of the neural crest arises between somites levels 1-5, migrates through or around these somites, and through pharyngeal arches 3-6 to the aortic sac. Due to its slow proliferation the distal part of the myocardial outflow tract becomes relatively shorter than its proximal part. Up to and including CS13, the distal myocardial boundary reaches to the pericardial reflection, with a thick acellular layer of endocardial jelly surrounding the lumen of the outflow tract. At CS14, the cells derived from the cardiac neural crest and columns of non-myocardial mural cells appear as new structures that transform the architecture of the aortic sac and the distal outflow tract.
Cells derived from the neural crest, which surround the arteries of the pharyngeal arches, begin to indent the dorsal wall of the aortic sac. They form a protrusion between its cranial portion, which connects to the arteries of the 3rd and 4th pharyngeal arches, and its caudal portion, which connects to the arteries of the 6th pharyngeal arch. The neural crest cells extend ventrally, having embraced the aortic sac bilaterally, and from there invade the endocardial jelly of the outflow tract as prongs of dense mesenchyme. In this way, they remodel the cuff of endocardial jelly into right- and left-sided columns. Meanwhile, endocardial cells that undergo epithelio-mesenchymal transformation also populate the endocardial jelly. The initially more numerous neural crest cells are necessary for correct positioning of the ridges, and for patterning of the arterial valvar leaflets. The feature, therefore, that distinguishes these ridges from the endocardial cushions of the atrioventricular canal is the presence of neural crest cells. For this reason, we describe the outflow entities as ridges, rather than cushions. The prongs within the ridges take a clockwise-spiraling course when observed in downstream direction, occupying septal and parietal locations at their junction with the developing right ventricle.
The cranial second heart field produces 2 waves of progenitor cells that are destined to form the outflow tract. The first wave arises at CS10, and contributes to the cranial wall of the muscular outflow tract until CS14 and to the ascending aorta thereafter. The second wave evolves more gradually between CS11 and CS15, and contributes to the caudal wall of the muscular outflow tract and, after CS14, to the pulmonary trunk. The cells of this second wave are dorsally continuous with, and probably originate from a phenotypically similar mass of pharyngeal mesenchyme surrounding the trachea This “club” of mesenchyme forms during CS13, and remains an identifiable entity during CS14 and CS15. The progenitor cells in the club converge and extend into a procession of cells that moves towards, and then into the relatively narrow outflow tract before locally differentiating. This convergent extension is mediated by the planar cell polarity pathway. When the addition of new cardiomyocytes ceases at CS14, non-myocardial cells start to form the distal portion of the outflow tract. These cells insert themselves cranially and caudally as columns between the remaining myocardial walls. Consequently, the distal myocardial boundary takes on a fishmouth appearance. In contrast to the caudal, or pulmonary, column, which extends to the peritracheal mesenchymal mass, the cranial, or aortic, column is short when traced into the pharyngeal floor.
In contrast to the neural crest cells, the cells of aortic and pulmonary mural columns do not penetrate the distal endocardial jelly, but maintain an oblique lateral-to-medial zone of apposition. The endocardial entities they abut are known as “swellings”. The cranial, or aortic, swelling differs from the caudal, or pulmonary swelling in that it is invaded by some neural crest cells. The swellings differ from the ridges in that they are initially (CS14 and CS15) confined to a small subsection of the middle portion of the outflow tract near the dog-leg bend. Consequently, the endocardial jelly, which still surrounds the lumen of the outflow tract as a smooth cuff at CS13, reorganizes distally into 4 orthogonal columns, while only two columns persist proximally.
Click an image to download a 3D-PDF. The file must be saved to a computer to enable the interactive options. The 3D-PDFs can be opened on any computer as long as Adobe PDF or equivalent reader is installed.
A 3D-PDF becomes activated by “clicking” with the mouse on the reconstruction.
A toolbar appears at the top of the screen that includes the option “model tree”.
The model tree displays a material list of structures in the upper box, and preset viewing options (cameras) in the lower box. The sequence of items corresponds to that in Supplemental Table 3 of the publication.
The list of visible structures can be modified by marking or unmarking a structure.
To manipulate the reconstruction, press the left mouse button to rotate it, the scroll button to zoom in or out, and the left and right mouse buttons simultaneously to move the embryo across the screen.
A structure can be rendered transparent by selecting that option from the drop-down menu after selecting the structure with the right mouse button.
To inspect a combination of structures, one is advised to build up the composition, beginning with a familiar component, such as a lumen, rather than deleting non-relevant structures one-by-one from a completely reconstructed specimen.
The slicer button in the toolbar allows making cross sections. The plane of section can be adjusted with the offset and tilt options.
The “loop wires” in Supplemental Figures 3-6 of the publication, which are drawn through the center of the endocardial heart tube, emphasize the changing shape of the heart loop during CS10-13.
The side length of the scale cubes is 200 μm.
The preset views correspond to the images shown in Figures 1-10 of the publication.
Note that items that are visible in these views can be altered by marking or unmarking a structure in the model tree.