
Back |
Interactive 3D PDF of Carnegie Stage 16 |
The interactive PDF below has been very kindly provided by Dr. Jill PJM Hikspoors, Prof. Wouter H Lamers,
Department of Anatomy & Embryology, Maastricht University, Maastricht, The Netherlands
Contact: jill.hikspoors@maastrichtuniversity.nl
Publication: 10.1038/s42003-022-03153-x
*Important* Although some web browsers will allow PDFs to be viewed online, the file must be downloaded and saved to a computer to enable the interactive options.
 |
 |
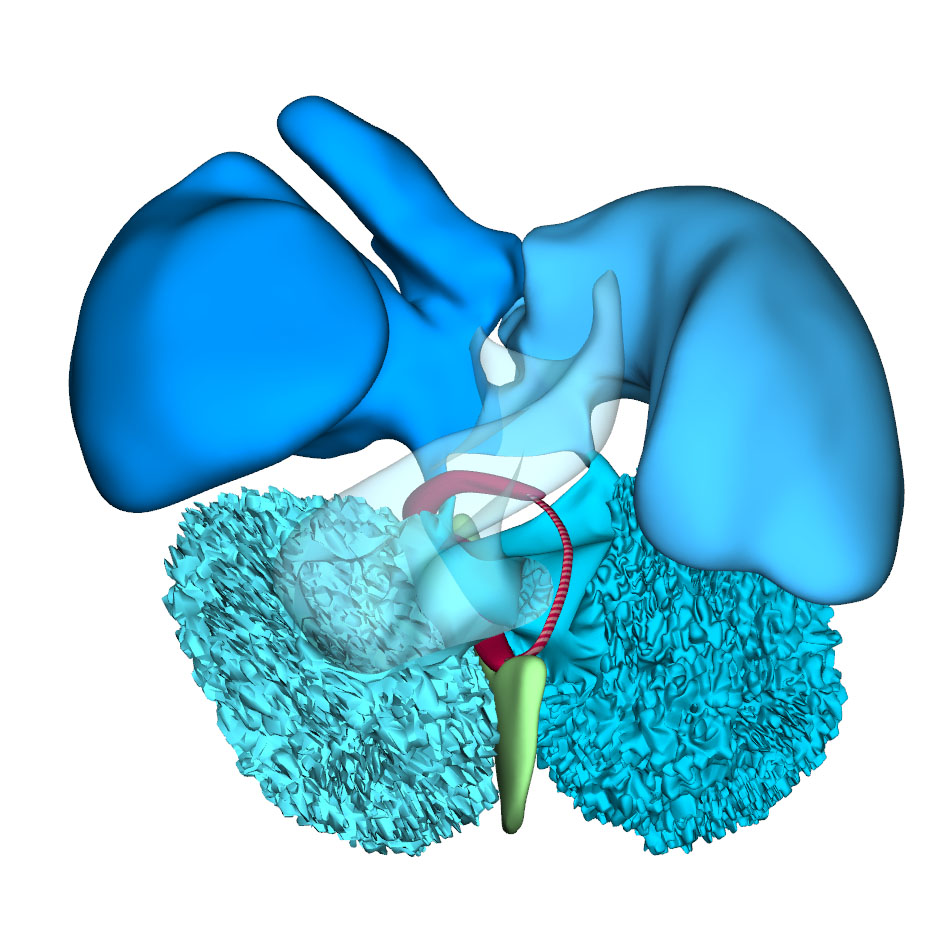 |
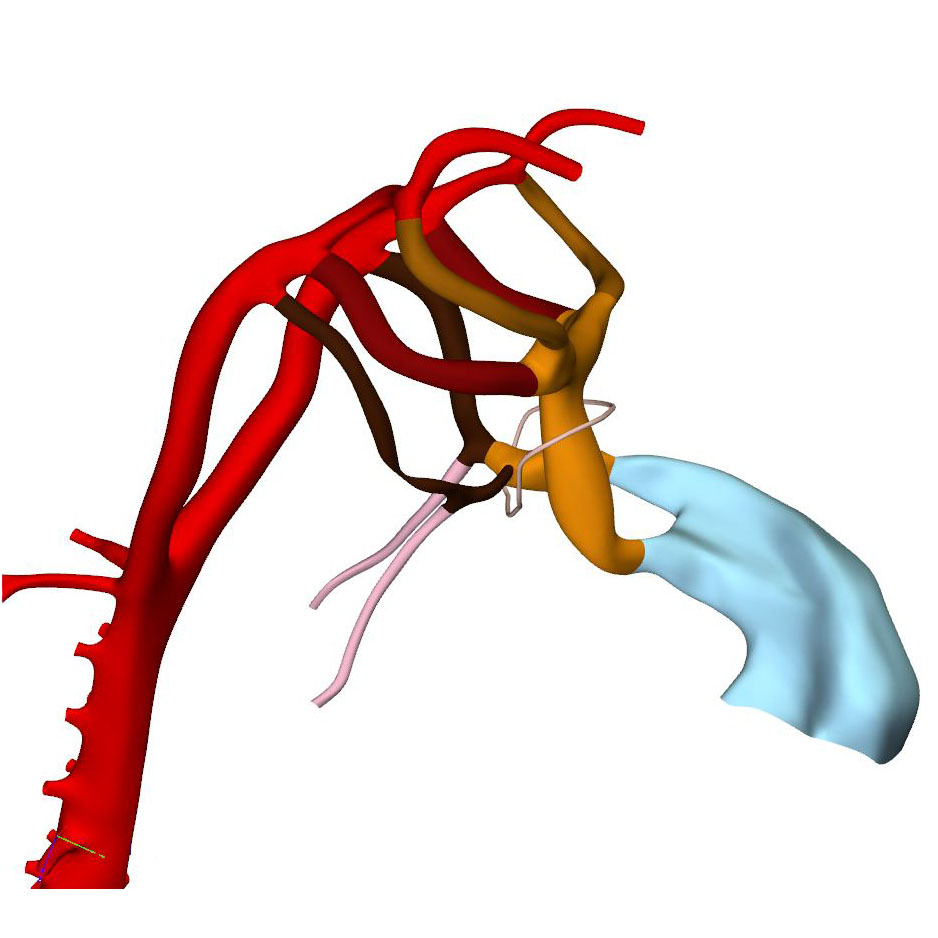 |
 |
||||
Right-sided view |
Right sagittal view: Interatrial foramen and interventricular foramen |
Cranial view: Lumen of the atria and ventricles |
Right ventral view: Asymmetrical pharyngeal arch arteries |
Left cranial view: Distal portion of ridges increase in size relative to their proximal counterpart to form arterial leaflets |
||||
Left cranial view: Columns with accompanied swellings are positioned inbetween ridges |
Left cranial view: Columns are located in the fish-mouth myocardial outflow tract |
Ventral view: changing position of the right ventricle |
Left cranial view: Position of the pulmonary and aortic channels |
This stage is reached at ~38 days after conception. The systemic venous sinus, its sinuatrial valves, and the sinus node have largely retained the appearances seen in the previous stage. The pulmonary vein remains a solitary and narrow channel. The primary atrial septum, with its mesenchymal cap, has extended further towards the atrioventricular canal. This reduces the size of the primary atrial foramen, but the atrioventricular cushions still have to fuse and a secondary atrial foramen still has to form in this embryo. This arrangement is seen in two CS16 embryos of the Blechschmidt collection, while a secondary atrial foramen has already formed in another embryo. In a 4th embryo, the atrioventricular cushions have fused, which brings about the closure of the primary atrial septum. The expansion of the atrial chambers to either side of the outflow tract reveals pronounced growth of the atrial appendages. With continuing caudal expansion of the atrium, the right border of the dorsal mesenchymal protrusion expands, like a spine, into the atrial cavity, growing between the atrial surfaces of the superior and inferior atrioventricular endocardial cushions.
The embryonic left and right ventricles are now of similar size and occupy a transverse plane. The changing boundaries of the interventricular foramen can still be followed conveniently in hearts stained for the GlN epitope. In the inner curvature of the heart, the right wall of the atrioventricular canal continues into the caudal part of the interventricular foramen. Rightward expansion of the confluent part of these structures across the muscular ventricular septum has produced a direct connection between the right atrium and the right ventricle. Subsequently, the cranial portion of the interventricular foramen will evolve into the channel between the left ventricle and the subaortic outlet. At this latter location, however, the primary ring (hatched section) has lost its expression of the GlN epitope, but remains identifiable as part of the central conduction system in birds. In the reconstructions, we have presumed that it lies, as in birds, in the inner curvature at the junction of the left ventricle with the outflow tract.
The length of the myocardial portion of the outflow tract increases ~4-fold in the 8 days between CS12, when it can be first differentiated from the embryonic right ventricle, and CS16, underscoring its continuous axial growth. The distal tongues of the myocardial outflow tract still extend close to the pericardial reflection, but relative to the diameter of the outflow tract, their length declines. Similarly, the aortic and pulmonary mural columns become relatively shorter. The ascending aorta and pulmonary trunk have increased substantially in length since their appearance at CS14, but axial growth of the pulmonary trunk ceases after CS16. This cessation of growth coincides with, and may reflect, the depletion of the peritracheal cell mass (“club”) that is present at CS14 and CS15.
At this stage of development, separate flows of blood reach the systemic and pulmonary arch arteries, but the endocardial outflow ridges have still to fuse mutually, and with the aortopulmonary septum. Hence, a narrow aortopulmonary foramen is still present distally between the subpulmonary and subaortic channels. The connections of the subaortic and subpulmonary channels with the ascending aorta and pulmonary trunk, respectively, now occupy left and right positions. This increasingly spiraling course of the intrapericardial arterial trunks corresponds in time with the unwinding of the spiraling course of the muscular outflow tract and its endocardial ridges. The still short swellings, which guard the narrow lumen of the developing arterial valves laterally, follow the unwinding course of the main endocardial ridges. Meanwhile, the distal part of the endocardial ridges of the outflow tract begins to increase in diameter relative to the proximal counterparts. This increase in size presages the remodeling of their distal surfaces into the arterial valvar leaflets during the next 2 stages. The carotid ducts have narrowed further. The artery of the right 6th arch is now narrower than the left one, in particular just distal to the origin of the right pulmonary artery. The diameter and perfusion of the left-sided dorsal aorta further increase.
Click an image to download a 3D-PDF. The file must be saved to a computer to enable the interactive options. The 3D-PDFs can be opened on any computer as long as Adobe PDF or equivalent reader is installed.
A 3D-PDF becomes activated by “clicking” with the mouse on the reconstruction.
A toolbar appears at the top of the screen that includes the option “model tree”.
The model tree displays a material list of structures in the upper box, and preset viewing options (cameras) in the lower box. The sequence of items corresponds to that in Supplemental Table 3 of the publication.
The list of visible structures can be modified by marking or unmarking a structure.
To manipulate the reconstruction, press the left mouse button to rotate it, the scroll button to zoom in or out, and the left and right mouse buttons simultaneously to move the embryo across the screen.
A structure can be rendered transparent by selecting that option from the drop-down menu after selecting the structure with the right mouse button.
To inspect a combination of structures, one is advised to build up the composition, beginning with a familiar component, such as a lumen, rather than deleting non-relevant structures one-by-one from a completely reconstructed specimen.
The slicer button in the toolbar allows making cross sections. The plane of section can be adjusted with the offset and tilt options.
The “loop wires” in Supplemental Figures 3-6 of the publication, which are drawn through the center of the endocardial heart tube, emphasize the changing shape of the heart loop during CS10-13.
The side length of the scale cubes is 200 μm.
The preset views correspond to the images shown in Figures 1-10 of the publication.
Note that items that are visible in these views can be altered by marking or unmarking a structure in the model tree.